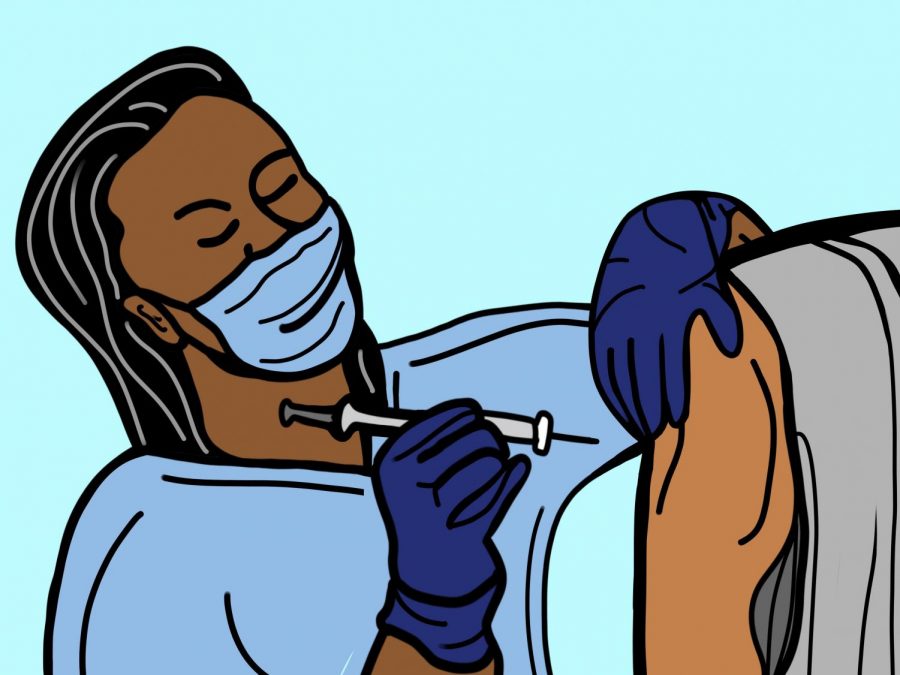
FDA approves booster vaccine dose for high risk populations
The Food and Drug Administration (FDA) approved the Booster Dose of Pfizer-BioNTech COVID-19 Vaccine for those age 65 and above and for 18 to 64 year olds who are at high risk for contracting COVID-19.
Visual Arts, ISIR and Performing Arts Teacher Ted Walch, who received the booster shot in August, said he felt no side effectsand is grateful to have received the dose early in the process.
“I honestly don’t have an opinion about whether or not students should receive the dose once it is approved for them,” Walch said. “I got it because, as a cancer survivor, I really don’t have a choice. I need to be doubly protected, so when Cedar Sinai called me and told me that I was at the top of their list as someone who needs to get the booster, I went to get it.”
Jane Hamilton ’22 said she originally felt relieved from the COVID-19 vaccination and is willing to receive the booster shot once available.
“I think that getting the vaccine made me feel a lot more protected from COVID-19 and was so crucial to stopping the spread,” Hamilton said. “Knowing that the vaccine doesn’t last forever, I think that the FDA approving the booster was super important. I would definitely get it once it is approved for my age group.”
Similarly, Jay Grosfeld ’23 said he would receive the dose once it is accepted for his age in hopes that the school might become maskless.
“I plan on getting it when it is approved for my age group, but I don’t really feel the need to get it right now just because of all the safety protocols at school,” Grosfeld said. “I hope that all of these efforts continue to reduce the spread because I hope for a normal school-year.”